
Kasha Dubaniewicz
Kasha is passionate about high-impact storytelling and believes in making positive changes that will lead to a better and happier world for all.
Why is pH a fundamental parameter of growing?
All plants need a balanced diet of nutrients (food) so that they can reach their full potential. The pH (potential Hydrogen) of your nutrient solution plays a pivotal role in making these elements available to your plants.

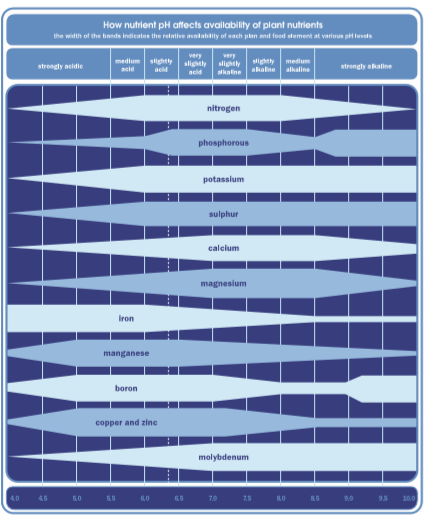
That’s because, in order to become available to your plants, your nutrients need to be soluble (dissolved in water) and have either a positive or negative electrical charge. When you measure pH, the value you see is based on ions, specifically hydrogen ions (H+). The amount of H+ determines the acidity or alkalinity; if there are more hydrogen ions present, then your pH will be acidic (0-7) and if there are less H+ relative to hydroxyl groups (OH-), the pH will be alkaline (7-14).
These hydrogen and hydroxyl ions play a very important role in nutrient availability. They can interact with and bind to nutrient ions, making them drop out of your solution in an insoluble form, meaning that your plant can’t recognise and absorb them. Since each nutrient ion has its own pH range within which it will be soluble, ions could become unavailable when your pH is outside this range. Known as nutrient lockout, this is a leading cause of nutrient deficiency, especially for nutrients such as iron and molybdenum.
That’s why it’s so important to regularly measure and adjust pH; without consistent management, nutrients can become insoluble and therefore unavailable to your plants, leading to nutrient lockout. Nutrient lockout isn’t easy to reverse; by measuring pH consistently, you’ll be able to spot and fix issues as they happen.
Featured product: Bluelab Multimedia pH Meter
How nutrient pH affects the availability of plant nutrients
How to measure pH in different growing environments
Along with conductivity and temperature, pH is one of the fundamentals you need to keep track of so that your plants can thrive. Now that we know what pH is, here are some of the most common ways to measure this fundamental parameter – dependent on your growing environment.
Measuring pH in solution:
There are a few different ways to measure in solution, with the quickest being a handheld pH pen or probe. Alternatively, you can also send samples to a laboratory or use testing strips. Here’s where you should think about measuring your solution pH and the benefits of each location.
In the nutrient reservoir: When you have full control over pH here, you can prevent nutrient ions from becoming insoluble and unavailable to the plant.
Inline: This enables you to monitor exactly what your plants can receive.
From the run-off: Measurements here ensure that your root zone pH values are within range, which means that your plants can take up the nutrients they need.
Measuring pH in soil:
Although soil has a natural ability to prevent pH from shifting dramatically, that doesn’t mean that you won’t need to measure the pH of your soil.
Different plants will grow best in different soil pH values; therefore, knowing the pH value of your soil may impact what you choose to grow. In addition, measuring your root zone pH will help you prevent root damage due to acid burns.
Here are the four most common methods for measuring soil pH:
- Handheld pH pen or probe: This is simplest and quickest method for taking measurements directly in your plant’s root zone.
- 2:1 dilution method: Mix two parts of distilled water with one part of your soil mix. Wait for the solids to settle and then measure the pH of the liquid.
- Saturated Media Extract (SME): Add distilled water to a sample of your soil until it’s saturated. Stir and wait at least an hour, drain through a filter to separate liquid and solids and then measure the pH of the liquid.
- Leachate PourThru Method: Fill your soil container to saturation with your normal irrigation water. After the container has drained for an hour, place a saucer underneath. Then pour enough distilled water on the surface of the container to get 50 ml (1.5 fluid ounces) of leachate to come out of the bottom of the container; this leachate can then be measured.
Measuring pH in soil-less media:
Soil-less growing media include coco coir, perlite, vermiculite and peat. These media don’t have the natural ability to control pH, so it’s very important to manage pH throughout the growing cycle. Measuring pH in soil-less media is similar to measuring soil pH; using a multimedia pH pen or probe is the fastest method.
.jpg?width=5000&name=shutterstock_1028738140-large%20(1).jpg)
Ready to start measuring pH? Here are our top product recommendations:
- Bluelab pH Pen: This fast and accurate handheld pen will help you measure pH and temperature in solutions on the go.
- Bluelab Soil pH Pen: Use this accurate and robust handheld pen to measure pH and temperature in your root zone as well as solution.
- Bluelab Multimedia pH Meter: This reliable handheld meter will accurately measure the pH of your root zone and in solution.
- Bluelab Combo Meter: Measuring the parameters of pH, conductivity and temperature at the click of a button, this three-in-one meter will become your go-to growing companion.
- Bluelab Combo Meter Plus: With all the perks of the Combo Meter, the addition of the Bluelab Leap pH Probe will allow you to take lightning-fast pH measurements in your root zone.
If you’d like to read more about the effects of pH management, check out this report on the impact of pH.





Submit a Comment